The New UKCA (UK Conformity Assessed) Marking
The UKCA (UK Conformity Assessed) marking is a new UK product marking that will be used for goods being placed on the market in Great Britain (England, Wales and Scotland).
It covers most goods which previously required the CE marking.
Countdown to implementation
Grace Period
When does this new standard come into action?
The UKCA mark can be applied to products sold in the UK from 1st January 2021.
There will be a grace period of 1 year to allow manufacturers to update existing product labels accordingly.
During this grace period, it will be acceptable for products that are sold in the UK to only bear the CE mark.
From 01 January 2022, all products sold in the UK will be required to carry the UKCA mark.
Additionally, products sold in Northern Ireland requiring a UKCA mark one year later, on 1 January 2023.
In some cases you will need to apply the new UKCA marking to goods being sold in Great Britain immediately from 1 January 2021.**
You are encouraged to be ready to adopt the UKCA marking as soon as possible!
The CE marking will only be valid in Great Britain for areas where GB and EU rules remain the same. If the EU changes its rules and you CE mark your product on the basis of those new rules you will not be able to use the CE marking to sell in Great Britain even before 31 December 2021
When to use the UKCA Marking?
**You will need to use the new UKCA marking immediately after 1 January 2021 if all of the following apply. Your product:
- is for the market in Great Britain
- is covered by legislation which requires the UKCA marking
- requires mandatory third-party conformity assessment
- conformity assessment has been carried out by a UK conformity assessment body and you haven’t transferred your conformity assessment files from your UK body to an EU recognised body before 1 January 2021
This does not apply to existing stock, for example if your good was fully manufactured and ready to place on the market before 1 January 2021.
In these cases your good can still be sold in Great Britain with a CE marking even if covered by a certificate of conformity issued by a UK body.
Where to apply the UKCA Marking?
In most cases, you must apply the UKCA marking to the product itself or to the packaging. In some cases, it may be placed on the manuals or on other supporting literature. This will vary depending on the specific regulations that apply to the product.
The following general rules apply:
- UKCA markings must only be placed on a product by you as the manufacturer or your authorised representative
- when attaching the UKCA marking, you take full responsibility for your product’s conformity with the requirements of the relevant legislation
- you must only use the UKCA marking to show product conformity with the relevant UK legislation
- you must not place any marking or sign that may misconstrue the meaning or form of the UKCA marking to third parties
- you must not attach other markings on the product which affect the visibility, legibility or meaning of the UKCA marking
- the UKCA marking cannot be placed on products unless there is a specific requirement to do so in the legislation
Rules for using the UKCA image
You must make sure that:
- if you reduce or enlarge the size of your marking, the letters forming the UKCA marking must be in proportion to the version set out (see right)
- the UKCA marking is at least 5mm in height – *unless a different minimum dimension is specified in the relevant legislation
- the UKCA marking is easily visible, legible (from 1 January 2023 it must be permanently attached)
Product areas covered by the UKCA marking
- Toy safety
- Recreational craft and personal watercraft
- Simple pressure vessels
- Electromagnetic compatibility
- Non-automatic weighing instruments
- Measuring instruments
- Lifts
- ATEX
- Radio equipment
- Pressure equipment
- Personal protective equipment
- Gas appliances
- Machinery
- Outdoor noise
- Ecodesign
- Aerosols
- Low voltage electrical equipment
- Restriction of hazardous substances
Products covered by the UKCA marking but have some special rules:
- medical devices
- rail interoperability
- construction products
- civil explosives
What are the differences between UKCA and CE marking?
Many of the differences between the two systems are administrative in nature and reflect that UKCA only applies in the UK and only requires information in English. This simplifies some aspects, such as where the technical information must be kept and which language applies.
Other differences relate to the separation of UK conformity assessment bodies from the EU Notified Body System.
What aspects are not changing?
Many facets are the same; the scope of products covered, technical requirements (essential requirements, standards) and conformity assessment procedures will all initially be alike. If your product is sold in both the EU and the UK, the technical file to show that it meets these requirements will also be the same.
What is the specific UK legislation that needs to be followed?
To implement the new regime, the UK government has issued several Statutory Instruments to amend current legislation.
The main regulations are The Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019, which runs to 659 pages.
These regulations amend most of the UK CE marking regulations for products placed on the UK market and stipulate the UKCA mark.
Where a directive required CE marking and UK regulations were already detailed, the amendments are limited to:
- replacing the CE mark with the UKCA mark,
- limiting applicability to products for the UK market,
- changing references to Notified Bodies to Approved Bodies,
- changing language references to English.
What safety markings are required in other Countries?
Read our BLOG on it below
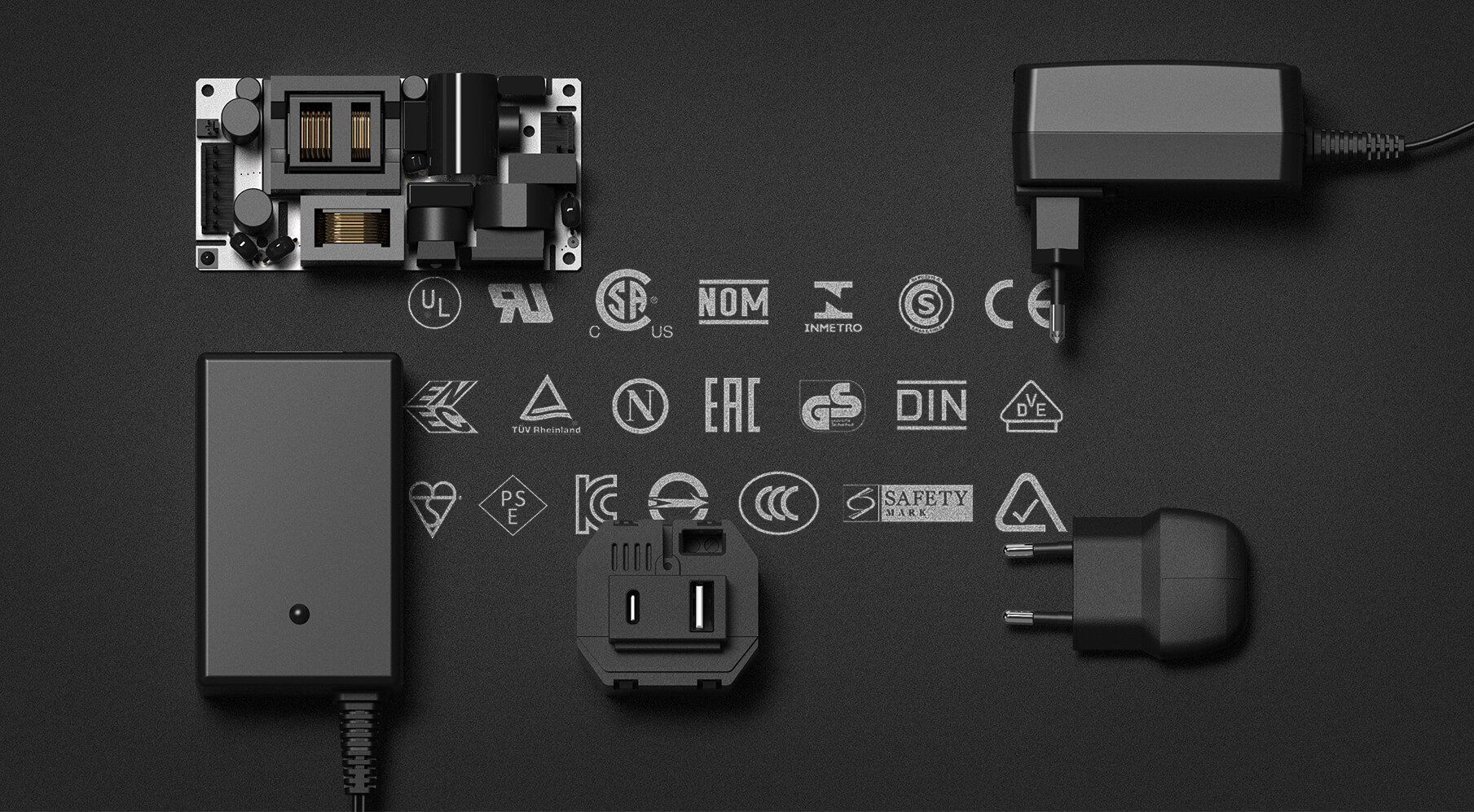
Be warned, one of the approvals listed is fake, can you spot it?!
-
BLOG: Know your standards!
Read it here!The sale and production of power supplies depends on the range of relevant safety standards in each individual region.
These standards are in place to protect each user from risks of fire, injury and electric shock.
Each and every power supply must meet the standards based in the area, and there are a high volume of standards, markings and agencies to understand...
CONTACT US
We will get back to you as soon as possible.
Please try again later.




CONTACT US
01423 796240
hello@haredata.co.uk
Unit 6 Stoneacre
St James Business Park
Grimbald Crag Close
Knaresborough
HG5 8PJ
FOLLOW US!
OPENING HOURS
- Mon - Thu
- -
- Friday
- -
- Sat - Sun
- Closed
©2024 Kastronix Limited trading as Haredata Electronics
Registered in England No: 5649420 VAT No: GB613440575